Substrate Concentration On Enzyme Activity
18.7: Enzyme Activity
- Page ID
- 16022
- To draw how pH, temperature, and the concentration of an enzyme and its substrate influence enzyme action.
The single nearly of import holding of enzymes is the ability to increase the rates of reactions occurring in living organisms, a property known equally catalytic action. Because well-nigh enzymes are proteins, their action is affected past factors that disrupt protein structure, as well as past factors that impact catalysts in full general. Factors that disrupt poly peptide structure include temperature and pH; factors that touch catalysts in full general include reactant or substrate concentration and catalyst or enzyme concentration. The activeness of an enzyme tin exist measured by monitoring either the charge per unit at which a substrate disappears or the rate at which a product forms.
Concentration of Substrate
In the presence of a given amount of enzyme, the rate of an enzymatic reaction increases as the substrate concentration increases until a limiting charge per unit is reached, later which further increment in the substrate concentration produces no meaning change in the reaction rate (role (a) of Figure \(\PageIndex{1}\)). At this point, so much substrate is nowadays that essentially all of the enzyme active sites have substrate leap to them. In other words, the enzyme molecules are saturated with substrate. The excess substrate molecules cannot react until the substrate already bound to the enzymes has reacted and been released (or been released without reacting).
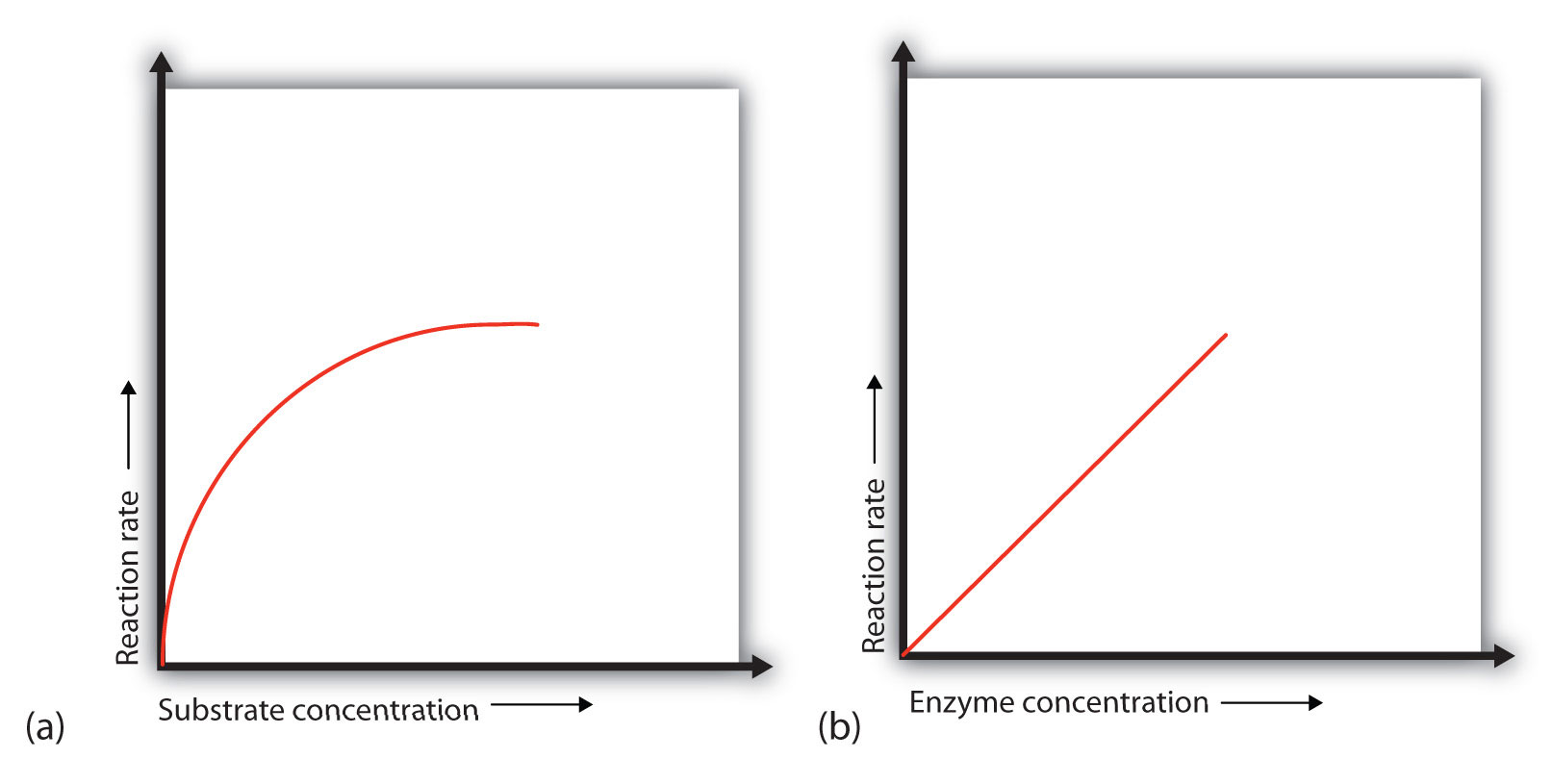
Let'due south consider an illustration. X taxis (enzyme molecules) are waiting at a taxi stand to accept people (substrate) on a 10-minute trip to a concert hall, one passenger at a fourth dimension. If only five people are present at the stand, the rate of their inflow at the concert hall is 5 people in x minutes. If the number of people at the stand is increased to 10, the rate increases to 10 arrivals in 10 minutes. With 20 people at the stand up, the charge per unit would still be 10 arrivals in 10 minutes. The taxis accept been "saturated." If the taxis could carry 2 or 3 passengers each, the same principle would apply. The rate would simply be higher (20 or 30 people in 10 minutes) before it leveled off.
Concentration of Enzyme
When the concentration of the enzyme is significantly lower than the concentration of the substrate (as when the number of taxis is far lower than the number of waiting passengers), the charge per unit of an enzyme-catalyzed reaction is straight dependent on the enzyme concentration (function (b) of Figure \(\PageIndex{1}\)). This is truthful for whatever catalyst; the reaction rate increases equally the concentration of the catalyst is increased.
Temperature
A general rule of thumb for most chemical reactions is that a temperature rise of 10°C approximately doubles the reaction charge per unit. To some extent, this dominion holds for all enzymatic reactions. Subsequently a certain bespeak, however, an increase in temperature causes a subtract in the reaction charge per unit, due to denaturation of the protein structure and disruption of the active site (function (a) of Figure \(\PageIndex{2}\)). For many proteins, denaturation occurs between 45°C and 55°C. Furthermore, even though an enzyme may appear to accept a maximum reaction rate between xl°C and fifty°C, near biochemical reactions are carried out at lower temperatures because enzymes are non stable at these higher temperatures and will denature afterwards a few minutes.
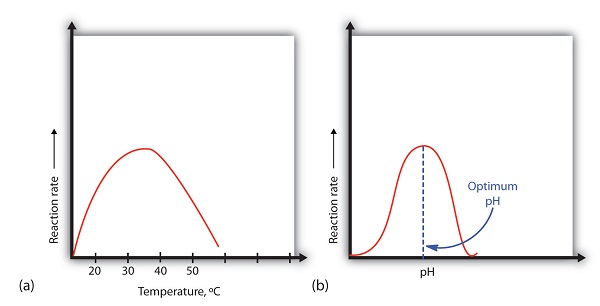
At 0°C and 100°C, the charge per unit of enzyme-catalyzed reactions is nearly zero. This fact has several applied applications. Nosotros sterilize objects by placing them in boiling h2o, which denatures the enzymes of any leaner that may be in or on them. We preserve our food past refrigerating or freezing it, which slows enzyme activeness. When animals go into hibernation in winter, their body temperature drops, decreasing the rates of their metabolic processes to levels that can be maintained by the amount of free energy stored in the fat reserves in the animals' tissues.
Hydrogen Ion Concentration (pH)
Because well-nigh enzymes are proteins, they are sensitive to changes in the hydrogen ion concentration or pH. Enzymes may be denatured by extreme levels of hydrogen ions (whether loftier or depression); any change in pH, even a small i, alters the degree of ionization of an enzyme's acidic and basic side groups and the substrate components likewise. Ionizable side groups located in the agile site must take a certain charge for the enzyme to bind its substrate. Neutralization of even ane of these charges alters an enzyme's catalytic activity.
An enzyme exhibits maximum activeness over the narrow pH range in which a molecule exists in its properly charged form. The median value of this pH range is called the optimum pH of the enzyme (function (b) of Effigy \(\PageIndex{2}\)). With the notable exception of gastric juice (the fluids secreted in the tummy), most body fluids have pH values between six and 8. Not surprisingly, well-nigh enzymes exhibit optimal activity in this pH range. However, a few enzymes have optimum pH values outside this range. For example, the optimum pH for pepsin, an enzyme that is active in the tummy, is 2.0.
Summary
Initially, an increase in substrate concentration leads to an increase in the rate of an enzyme-catalyzed reaction. As the enzyme molecules become saturated with substrate, this increase in reaction charge per unit levels off. The rate of an enzyme-catalyzed reaction increases with an increase in the concentration of an enzyme. At depression temperatures, an increment in temperature increases the rate of an enzyme-catalyzed reaction. At college temperatures, the poly peptide is denatured, and the charge per unit of the reaction dramatically decreases. An enzyme has an optimum pH range in which it exhibits maximum action.
Substrate Concentration On Enzyme Activity,
Source: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Basics_of_General_Organic_and_Biological_Chemistry_%28Ball_et_al.%29/18:_Amino_Acids_Proteins_and_Enzymes/18.07:_Enzyme_Activity
Posted by: goldmanhocush64.blogspot.com


0 Response to "Substrate Concentration On Enzyme Activity"
Post a Comment