Why Is It Necessary Use A Pencil When Writing On A Tlc Plate?
Sparse Layer Chromatography
- Page ID
- 2047
Sparse layer chromatography (TLC) is a chromatographic technique used to separate the components of a mixture using a sparse stationary stage supported past an inert backing. It may exist performed on the analytical scale as a ways of monitoring the progress of a reaction, or on the preparative scale to purify minor amounts of a compound. TLC is an analytical tool widely used because of its simplicity, relative low cost, loftier sensitivity, and speed of separation.TLC functions on the same principle as all chromatography: a chemical compound will have different affinities for the mobile and stationary phases, and this affects the speed at which it migrates. The goal of TLC is to obtain well divers, well separated spots.
Retentivity Factor
Later a separation is complete, individual compounds appear as spots separated vertically. Each spot has a memory factor (Rf) which is equal to the distance migrated over the full altitude covered by the solvent. The \( R_f\) formula is
\[ R_f= \dfrac{\text{distance traveled past sample}}{\text{distance traveled by solvent}} \]
The \( R_f\) value can exist used to identify compounds due to their uniqueness to each compound. When comparing ii different compounds nether the same atmospheric condition, the compound with the larger \( R_f\) value is less polar because information technology does not stick to the stationary phase as long as the polar chemical compound, which would accept a lower \( R_f\) value.
\( R_f\) values and reproducibility can be affected by a number of unlike factors such as layer thickness, wet on the TLC plate, vessel saturation, temperature, depth of mobile phase, nature of the TLC plate, sample size, and solvent parameters. These effects normally cause an increment in \( R_f\) values. However, in the example of layer thickness, the \( R_f\) value would decrease considering the mobile phase moves slower upward the plate.
If it is desired to express positions relative to the position of another substance, x, the \( R_x\) (relative retention value) can be calculated:
\[ R_x= \dfrac{\text{distance of chemical compound from origin}}{\text{distance of compound x from origin}} \]
While \(R_f\) can never be greater than i, \( R_x\) tin can exist (i.eastward., faster than the reference chemical compound \(ten\).
Apparatus
Plates (Stationary Phase)
As stated earlier, TLC plates (likewise known as chromatoplates) tin be prepared in the lab, but are most commonly purchased. Silica gel and alumina are among the most common stationary phases, but others are available besides. Many plates contain a chemical compound which fluoresces under short-moving ridge UV (254 nm). The backing of TLC plates is often composed of glass, aluminum, or plastic. Glass plates are chemically inert and best withstand reactive stains and rut, simply are brittle and tin can be difficult to cut. Aluminum and plastic plates can be cut with scissors, only aluminum may non withstand strongly acidic or oxidizing stains, and plastic does not withstand the high heat required to develop many stains. Aluminum and plastic plates are too flexible, which may outcome in flaking of the stationary phase. Never nether whatsoever circumstances touch the face of a TLC plate with your fingers as contagion from peel oils or residues on gloves can obscure results. Instead, always handle them by the edges, or with forceps.
The backdrop of your sample should be considered when selecting the stationary phase. As shown below in Table \(\PageIndex{1}\), silica gel can be exclusively used for amino acids and hydrocarbons. It is as well important to note that silica gel is acidic. Therefore, silica gel offers poor separation of bones samples and tin crusade a deterioration of acid-labile molecules. This would be truthful for alumina plates in acidic solutions equally well. It is of import to note that there are differences between silica gel and alumina. Alumina is bones and information technology will not separate sample sizes every bit large as silica gel would at a given layer thickness. Also, alumina is more chemically reactive than silica gel and equally a outcome, would require more care of compounds and compound classes. This care would avoid decomposition and rearrangement of the sample.
| Stationary Phase | Chromatographic Machinery | Typical Application |
|---|---|---|
| Silica Gel | adsorption | steroids, amino acids, alcohols, hydrocarbons, lipids, aflaxtoxin, bile, acids, vitamins, alkaloids |
| Silica Gel RP | reversed phase | fat acids, vitamins, steroids, hormones, carotenoids |
| Cellulose, kieselguhr | sectionalization | carbohydrates, sugars, alcohols, amino acids, carboxylic acids, fat acids |
| Aluminum oxide | adsorption | amines, alcohols, steroids, lipids, aflatoxins, bile acids, vitamins, alkaloids |
| PEI cellulose | ion substitution | nucleic acids, nucleotides, nucelosides, purines, pyrimidines |
| Magnesium silicate | adsorption | steroids, pesticides, lipids, alkaloids |
Chromatographic Columns is a good reference to learn more most the dissimilar types of columns and stationary phases.
Solvent (Mobile Phase)
Proper solvent selection is peradventure the about important aspect of TLC, and determining the best solvent may require a degree of trial and mistake. Every bit with plate selection, keep in heed the chemical properties of the analytes. A common starting solvent is 1:1 hexane:ethyl acetate. Varying the ratio can take a pronounced effect of \(R_f\). \(R_f\) values range from 0 to 1 with 0 indicating that the solvent polarity is very low and 1 indicating that the solvent polarity is very high. When performing your experiment, yous do not want your values to be 0 or ane because your components that yous are separating have different polarities. If the value is 0, y'all need to increment your solvent polarity considering the sample is non moving and sticking to the stationary phase. If the value is ane, you need to decrease your solvent polarity because the compound was non able to separate.
If you lot know that ane component of a mixture is insoluble in a given solvent, but another component is freely soluble in it, it oft gives good separations. How fast the compounds travel upward the plate depends on two things:
- If the compound is soluble in the solvent, information technology will travel farther up the TLC plate
- How well the compound likes the stationary stage. If the compound likes the stationary phase, information technology volition stick to it, which will crusade it to not move very far on the chromatogram.
Y'all should be able to determine which by looking at the \(R_f\) value.
Acids, bases, and strongly polar compounds frequently produce streaks rather than spots in neutral solvents. Streaks go far difficult to calculate an \(R_f\) and may occlude other spots. Adding a few percent of acetic or formic acid to the solvent can correct streaking with acids. Similarly for bases, adding a few percent triethylamine can improve results. For polar compounds adding a few percent methanol can likewise ameliorate results.
The volatility of solvents should also exist considered when chemical stains are to exist used. Any solvent left on the plate may react with the stain and conceal spots. Many solvents can be removed by allowing them to sit down on the bench for a few minutes, but very nonvolatile solvents may require fourth dimension in a vacuum chamber. Volatile solvents should only exist used one time. If the mobile phase is used repeatedly, results will not be consistent or reproducible.
Useful Solvent Mixtures
- A solvent that can exist used for separating mixtures of strongly polar compounds is ethyl acetate : butanol : acetic acrid : water, 80:10:5:5.
- To separate strongly basic components, make a mixture of 10% NH4OH in methanol, and then brand a 1 to 10% mixture of this in dichlormethane.
- Mixtures of x% methanol or less in DCM can be useful for separating polar compounds.
Pipettes
- Spots are applied to the plate using very sparse drinking glass pipettes. The capillary should exist thin enough to use a neat spot, but not so thin as to foreclose the uptake of an adequate quantity of analyte. Here is a popular method of producing TLC pipettes.
- Heat a glass capillary in the very tip of a Bunsen burner flame just until it becomes pliable and then pull the ends autonomously until the center of the capillary is significantly narrower. Snap this in half and use the thin end to use spots.
Spotting and Developing
Developing a TLC plate requires a developing bedroom or vessel. This tin exist equally simple as a wide-oral fissure jar, merely more than specialized pieces of glassware to conform large plates are available. The chamber should contain enough solvent to only encompass the lesser. Information technology should also contain a piece of filter paper, or other absorbent cloth to saturate the atmosphere with solvent vapors. Finally, it should have a chapeau or other covering to minimize evaporation.
- Cut the plate to the correct size and using a pencil (never always use a pen), gently draw a straight line across the plate approximately 1 cm from the bottom. Exercise not use excessive forces when writing on a TLC plate equally this will remove the stationary phase. It is important to use a pencil rather than a pen because inks usually travel up the plate with the solvent. An example of how black ink separates is shown in the section labeled "examples".
- Using TLC pipettes, utilise spots of analyte to the line. Make sure enough sample is spotted on the plate. This tin can be done by using the short-wave UV. A imperial spot should be seen. If the spot is not visible, more sample needs to be applied to the plate. If a standard of the target compound is available, it is good practice to produce a co-spot past spotting the standard onto a spot of the unknown mixture. This ensures the identity of the target compound.
- Identify the plate into the chamber as evenly as possible and lean it against the side. Never let the bulk solvent to ascent above the line yous drew. Allow capillary action to draw the solvent upwardly the plate until it is approximately 1 cm from the end. Never allow the solvent to drift all the way to the end of the plate.
- Remove the plate and immediately draw a pencil line beyond the solvent front.
- Use a brusk-wave UV light and circumvolve the components shown with a pencil.
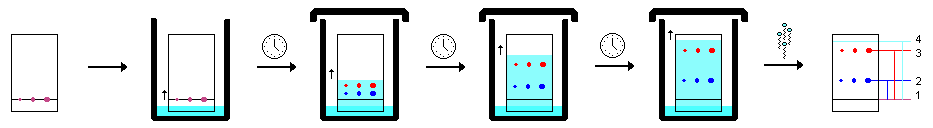
Visualizing
If fluorescent plates are used, a number of compounds can be seen by illuminating the plate with curt-moving ridge UV. Quenching causes night spots on the surface of the plate. These night patches should exist circled with a pencil. For compounds which are non UV active, a number of chemical stains tin can exist used. These tin exist very general, or they can exist specific for a item molecule or functional group.
Iodine is amid the most common stains. Plates are placed in a jar containing iodine crystals, or covered in silica gel with iodine dispersed throughout, for approximately 1 minute. Most organic compounds will exist temporarily stained chocolate-brown. Some popular general apply stains are Permanganate, ceric ammonium molybdate (CAM), and p-anisaldehyde. These tin can be kept in jars which plates are dipped into, or in spray bottles.
To develop a plate with permanganate, spray or dip the plate and heat information technology with a heat-gun. Agree the plate confront upward 10 to 20 cm higher up the heat gun until the bulk water evaporates. And so motion the plate to 5 to 10 cm above the heat gun and heat it until white/yellow/brown spots appear. Overheating will turn the entire plate brownish, obscuring the spots. If glass plates are used it is oft easier to run into spots through the backing because it is harder to overheat. CAM and p-anisaldehyde stained plates are adult similarly. Overheating CAM stained plates turns everything bluish.
Common Problems in TLC
There are common problems in TLC that should exist avoided. Ordinarily, these bug can be solved or avoided if taught proper techniques.
- Over-large Spotsouth: Spotting sizes of your sample should exist not be larger than 1-ii mm in bore. The component spots will never be larger than or smaller than your sample origin spot. If you take an over-big spot, this could cause overlapping of other component spots with similar \(R_f\) values on your TLC plate. If overlapping occurs, information technology would testify hard to resolve the dissimilar components.
- Uneven Advance of Solvent Front: Uneven advance of the mobile phase is a common problem encountered in TLC. Consequences would be inaccurate Rf values due to the uneven accelerate of sample origin spots. This uneven advance tin be caused past a few factors listed beneath.
- No apartment bottom. When placing the TLC plate into the bedroom, place the bottom of the plate on the edge of the bedroom (normally glass container (e.grand. beaker)) and lean the top of the plate along the other side of the chamber. Also, make sure that the TLC plate is placed in the sleeping accommodation evenly. Practise non tilt the plate or sit it at an angle.
- Not enough solvent. There should be plenty solvent (depends on size of the bedroom) to travel up the length of the TLC plate.
- Plate is not cut evenly. Information technology is recommended that a ruler is used so that the plate is cut evenly.
Rarely, water is used as a solvent because information technology produces an uneven curve front which is mainly accounted for by its surface tension.
- Streaking : If the sample spot is too concentrated, the substance will travel up the stationary stage every bit a streak rather than a single separated spot. In other words, the solvent tin non handle the concentrated sample and in result, moves as much of the substance as it tin up the stationary phase. The substance that information technology can not motility is left behind. This tin be eliminated by diluting the sample solution. To ensure that y'all take enough solution, use a short-wave UV light to see if the spot is visible (normally royal in color), as stated earlier.
- Spotting : The sample should be to a higher place the solvent level. If the solvent level covers the sample, the sample spot will exist washed off into the solvent before it travels up the TLC plate. An example is shown below.
Example: Analyzing Commercial Analgesics
Sparse layer chromatography of 3 analgesics and caffeine under U.V. light was carried out in lodge to bear witness the separation taking place. It is non a recommended technique in the laboratory. Due to the nature of the uv hazard polycarbonate safety glasses (which absorb short wavelength U.Five. light) and rubber gloves were worn throughout.
V samples were run on a single TLC plate. The samples were (left to right on the plate):
- Ibuprofen (ICU)
- caffeine (CAF)
- u? = a commercial 'hurting relief' medicine, used as an unknown
- Acetominophen (PAR)
- Aspirin (ASP)

The samples were dissolved in ethanol for spotting onto the plate. The TLC plate was run in an open beaker under short wavelength u.v. light using ethyl ethanoate as the eluting solvent.
.gif?revision=1)
The movement of the dark purple spots (samples) during the running of the plate can be observed in the animation. The original movie tin exist viewed here.

It is easy to encounter which are the 2 active ingredients in the unknown commercial pain relief medicine by comparison of the spots with the standard reference materials running on either side (caffeine and acetominophen).
Advantages and Disadvantages of TLC
TLC is very elementary to utilise and cheap. Undergraduates can be taught this technique and apply its similar principles to other chromatographic techniques. There are little materials needed for TLC (chamber, watch glass, capillary, plate, solvent, pencil, and UV-calorie-free). Therefore, once the best solvent is found, information technology can be practical to other techniques such every bit High functioning liquid chromatography. More than 1 chemical compound can be separated on a TLC plate every bit long as the mobile phase is preferred for each compound. The solvents for the TLC plate can be changed easily and it is possible to use several different solvents depending on your desired results. Equally stated before, TLC tin can be used to ensure purity of a compound. It is very easy to bank check the purity using a UV-light. The identification of almost compounds tin can be done simply past checking \( R_f\) literature values. Y'all tin modify the chromatography weather condition easily to increase the optimization for resolution of a specific component.
TLC plates do not have long stationary phases. Therefore, the length of separation is express compared to other chromatographic techniques. Also, the detection limit is a lot college. If you would need a lower detection limit, 1 would have to use other chromatographic techniques. TLC operates every bit an open arrangement, so factors such as humidity and temperature can exist consequences to the results of your chromatogram.
References
- Touchstone, Joseph C. Practice of thin layer chromatography. 2nd ed. New York: Wiley, 1983.Print.
-
Geiss, Friedrich. Fundamentals of sparse layer chromatography planar chromatography. Heidelberg: A. Hüthig, 1987. Print.
-
Touchstone, Joseph C. Practice of sparse layer chromatography. third ed. New York: Wiley, 1992. Print.
Problems and Solutions
Figure 3: TLC plate nether UV calorie-free with values for altitude traveled of solvent and components.
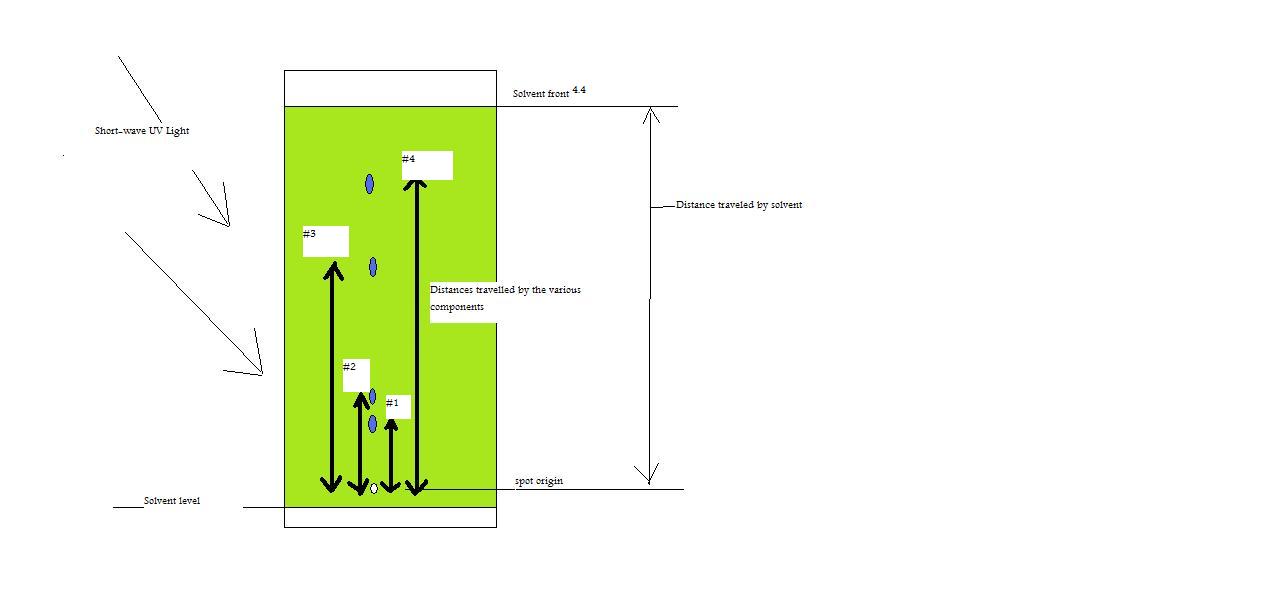
Given:
#1=1.4 cm
#2= one.five cm
#three= iii.one cm
#four= 3.half-dozen cm
Using only the given data and the above effigy, answer the issues listed below.
- What is the Rf value for component #2?
- What is the Rf value for component # 3?
- What is the relative memory value for components #one and # four, with # 4 being compound x?
- Using the answers from questions 1 and ii and bold that components ii and 3 are dissimilar compounds, which component would be considered more polar? Explain.
Answers
- ane.five/4.iv=0.34
- 3.1/iv.4=0.lxx
- ane.4/3.half-dozen=0.39
- Component # 2 would be considered more polar because it has the lower Rf value, which means that it sticks to the stationary phase a lot stronger than component #iii and therefore moves slower in the mobile phase.
Why Is It Necessary Use A Pencil When Writing On A Tlc Plate?,
Source: https://chem.libretexts.org/Ancillary_Materials/Demos_Techniques_and_Experiments/General_Lab_Techniques/Thin_Layer_Chromatography#:~:text=Do%20not%20use%20excessive%20forces,the%20plate%20with%20the%20solvent.
Posted by: goldmanhocush64.blogspot.com


0 Response to "Why Is It Necessary Use A Pencil When Writing On A Tlc Plate?"
Post a Comment